Now that's the way you do it!
One of the overarching themes of this blog has been skepticism in the claims of alternative medicine. Consequently, a recurring type of post has been the debunking of some claim or other made by the proponents of alternative medicine. Sometimes debunking these claims is like shooting fish in a barrel, allowing for humorous play with the concept, and sometimes the claims are a bit harder to debunk, requiring a more serious approach. In the end, however, the vast majority of altie claims turn out to be without a solid basis in science and without evidence of efficacy if you look at them with a critical eye. From these posts, some conclude that I'm hoplessly hostile and biased against alternative medicine, but such is not the case at all. I merely insist that the claims of alternative medicine advocates be subjected to the same scientific standards as conventional medicine .
It is not at all uncommon for cancer patients to ask if there are any dietary manipulations that will treat their cancer. My stock response is usually that, although there is evidence for the efficacy of various dietary manipulations in reducing one's risk of cancer, there is little or no good evidence that dietary manipulations will treat a cancer once it's become established. By the time a patient develops a detectable cancer requiring treatment, the horse has left the barn a long time ago, so to speak. Consequently, other than making sure one eats a standard good diet with adequate calories, lots of fruits and vegetables, low fat, high fiber, etc., there are no known specific diets that will improve a cancer patient's chances of beating the disease, at least none with any good evidence to support it. However, a recent study out of the Princess Margaret Hospital Cancer Center and the Massachusetts General Hospital Cancer Center may provide the basis for suggesting one dietary manipulation that might ultimately be useful in treating breast cancer, if subsequent studies pan out.
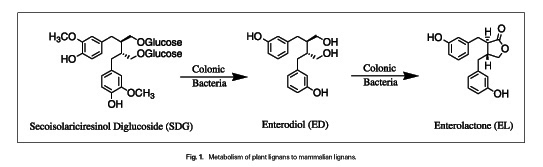
It has been known for a while that lignans have antiestrogenic properties, and blocking estrogen has been the mainstay of breast cancer treatment for estrogen receptor-positive tumors for decades. There are also preclinical studies indicating that lignans may have antitumor properties. It turns out that flaxseed is one of the richest sources of mammalian lignan precursors such as secoisolariciresinol diglucoside (SDG). These lignan precursors are converted by the bacteria in the colon into enterodiol (ED) and enterolactone (EL), the active lignans as shown below:
There is also preclinical evidence that putting flaxseed in the diet can inhibit the growth of tumors in mice and even potentiate the efficacy of the estrogen blocker Tamoxifen. Based on this, the investigators decided to test whether this might be true in humans; so they designed a clinical trial, and you're going to love how they did it. First off (this isn't the part you're going to love, but it has to be described), they designed the trial the same way many neoadjuvant therapy trials. Thirty-two postmenopausal women with a needle biopsy-proven cancer were randomized to two groups, one of which would receive the treatment and the other the placebo for 30-40 days prior to definitive surgery, with measurements of tumor markers of aggressiveness measured in the first pretreatment biopsy and then again in the definitive resection. Note that this is the sort of study that could only be done in a country with a national health care system. In the U.S., it would be difficult indeed to persuade women recently diagnosed with to wait 30-40 days for their definitive surgery. In Canada, though, that's the usual waiting time; so the investigators could quite reasonably use the argument that their therapy was not unduly delaying definitive surgery.
Now here's the part you're going to love. The way they decided to administer flaxseed or placebo was to bake it into muffins that women were to eat every day. From the Methods section of the paper:
I can assure you that I've never seen a section in a Methods section of a scientific paper entitled "Study Muffins" before. My partners and I were discussing this article at journal club and we couldn't help but have a healthy chuckle and make all sorts of jokes about "studmuffins" and "magic muffins." It didn't help that the first sentence in the Results section read:
All kidding aside, though, this was a pretty well-designed prospective randomized, double-blind, placebo-controlled pilot trial to look at what effect consuming flaxseed oil had on tumor markers in breast cancer. They measured urinary lignan excretion to show that it went up in the experimental group, meaning that lignans were being made, and they controlled for caloric and micronutrient intake between groups. What they found is as follows:
Another important implication of this study is that, contrary to what some alties will tell you, it is possible to evaluate alternative medical claims in well-designed, well-controlled scientific studies. As I have pointed out before, the vast majority of these claims will not stand up to the light of careful scientific scrutiny, but it is possible that, in this one case, one claim for one alternative medicine intervention will.
And this is the way you start to do find out which of these claims might have something behind them.
It is not at all uncommon for cancer patients to ask if there are any dietary manipulations that will treat their cancer. My stock response is usually that, although there is evidence for the efficacy of various dietary manipulations in reducing one's risk of cancer, there is little or no good evidence that dietary manipulations will treat a cancer once it's become established. By the time a patient develops a detectable cancer requiring treatment, the horse has left the barn a long time ago, so to speak. Consequently, other than making sure one eats a standard good diet with adequate calories, lots of fruits and vegetables, low fat, high fiber, etc., there are no known specific diets that will improve a cancer patient's chances of beating the disease, at least none with any good evidence to support it. However, a recent study out of the Princess Margaret Hospital Cancer Center and the Massachusetts General Hospital Cancer Center may provide the basis for suggesting one dietary manipulation that might ultimately be useful in treating breast cancer, if subsequent studies pan out.
It has been known for a while that lignans have antiestrogenic properties, and blocking estrogen has been the mainstay of breast cancer treatment for estrogen receptor-positive tumors for decades. There are also preclinical studies indicating that lignans may have antitumor properties. It turns out that flaxseed is one of the richest sources of mammalian lignan precursors such as secoisolariciresinol diglucoside (SDG). These lignan precursors are converted by the bacteria in the colon into enterodiol (ED) and enterolactone (EL), the active lignans as shown below:
There is also preclinical evidence that putting flaxseed in the diet can inhibit the growth of tumors in mice and even potentiate the efficacy of the estrogen blocker Tamoxifen. Based on this, the investigators decided to test whether this might be true in humans; so they designed a clinical trial, and you're going to love how they did it. First off (this isn't the part you're going to love, but it has to be described), they designed the trial the same way many neoadjuvant therapy trials. Thirty-two postmenopausal women with a needle biopsy-proven cancer were randomized to two groups, one of which would receive the treatment and the other the placebo for 30-40 days prior to definitive surgery, with measurements of tumor markers of aggressiveness measured in the first pretreatment biopsy and then again in the definitive resection. Note that this is the sort of study that could only be done in a country with a national health care system. In the U.S., it would be difficult indeed to persuade women recently diagnosed with to wait 30-40 days for their definitive surgery. In Canada, though, that's the usual waiting time; so the investigators could quite reasonably use the argument that their therapy was not unduly delaying definitive surgery.
Now here's the part you're going to love. The way they decided to administer flaxseed or placebo was to bake it into muffins that women were to eat every day. From the Methods section of the paper:
Study muffins. Study muffins were prepared in the standard manner by Canada Bread Co. (Toronto, ON, Canada). They contained similar ingredients and were prepared to contain 20.7 g white wheat flour for flaxseed muffins or 20.7 g whole-wheat flour for placebo muffins. Flaxseed muffins contained 25 g ground flaxseed. Placebo muffins were prepared with whole-wheat flour instead of white wheat flour to raise the dietary fiber content closer to that of the flaxseed muffins. All muffins were formulated to be isocaloric and equivalent in fat, protein, and dietary fiber. Hence additional canola oil (10 g) was added to placebo muffins but not to the flaxseed muffins. Muffins were also flavored with nutmeg, cinnamon, and vanilla extract to help maintain subject blindness. All flaxseed came from the same source (Omega Products, Melfort, Saskatchewan, Canada) and batch, and contained 2 mg of secoisolariciresinol diglucoside per gram. The patients kept their weekly supply of muffins at 20° C and defrosted them as needed. They ate one muffin per day at breakfast time. Any uneaten muffins or portions thereof were returned and weighed. The total intake of flaxseed was estimated as 25 g X treatment days - uneaten amounts.
Muffin intake compliance was good (95.4% in the placebo and 92.5% in the flaxseed group) and did not differ significantly between the groups."Muffin intake compliance"? I love it. I can't help but wonder if the investigators had a sense of humor and did that on purpose, knowing how it would sound.
All kidding aside, though, this was a pretty well-designed prospective randomized, double-blind, placebo-controlled pilot trial to look at what effect consuming flaxseed oil had on tumor markers in breast cancer. They measured urinary lignan excretion to show that it went up in the experimental group, meaning that lignans were being made, and they controlled for caloric and micronutrient intake between groups. What they found is as follows:
- A marker of tumor cell proliferation (Ki-67) decreased by 34.7% in the tumors in the experimental group compared to the control. Ki-67 reduction has been shown to be a pretty good surrogate endpoint biomarker for the efficacy of hormonal therapy in breast cancer. It is thought, but not yet proven mechanistically, that the mechanism of action of lignans is antiestrogenic. Given that estrogen-receptor tumors also demonstrated a reduction in Ki-67, it is clear that antiestrogenic effects alone cannot explain the action of flaxseed, and there are a number of hypotheses as to the mechanism of action of flaxseed.
- The c-erbB2 score (another marker of tumor cell proliferation and aggressiveness based on scoring the expression of the oncogene Her-2/neu, which is another name for c-erbB2) decreased by 71% in the control group compared to the control.
- Tumor cell apoptosis (programmed cell death) increased by 30.7% in the experimental group but remained the same in the control.
So, does this mean postmenopausal women with breast cancer should toss their Tamoxifen or Arimidex into the trash and start making flaxseed muffins? Absolutely not. Not on the basis of this small unconfirmed trial, it doesn't. This study needs to be confirmed in a larger number of women over a longer treatment period of time with definitive demonstration of actual tumor shrinkage. Biomarkers do not always correlate with antitumor response. The more likely implication of this study is that this dietary manipulation might have utility as a strategy for breast cancer prevention, either in lieu of long term Tamoxifen or Arimidex or as an adjunct. There is also the problem of standardization common to herbal remedies or natural substance remedies like flaxseed that can make it difficult to make sure that every lot of flaxseed has the the same amount of active ingredient.The only side effects reported by subjects were increased abdominal fullness and bowel movements due to the high fiber content of flaxseed. As an increase in bowel movement may be desirable for patients with low fiber intake and chronic constipation, this effect may not be considered adverse compared to other side effects seen with breast cancer drugs such as tamoxifen or aromatase inhibitors.
Another important implication of this study is that, contrary to what some alties will tell you, it is possible to evaluate alternative medical claims in well-designed, well-controlled scientific studies. As I have pointed out before, the vast majority of these claims will not stand up to the light of careful scientific scrutiny, but it is possible that, in this one case, one claim for one alternative medicine intervention will.
And this is the way you start to do find out which of these claims might have something behind them.

I assume, however, that there would be no harm in adding flaxseed to the diet. At the very least, it sounds like an effective way to increase dietary fiber! I've never had breast cancer, but I am a woman over 30 who has not yet had a child, and I know that increases my chances of getting breast cancer. I'll be interested to see how this pans out!
ReplyDelete-Sylvanite
Interesting post, I haven't seen anything about it in the news.
ReplyDeleteYou've got one small typo when you're listing the results of the study:
2. The c-erbB2 score (another marker of tumor cell proliferation and aggressiveness based on scoring the expression of the oncogene Her-2/neu, which is another name for c-erbB2) decreased by 71% in the control group compared to the control.
I assume you mean "decreased 71% in the *experimental* group compared to the control."
-Matt
Very interesting study ... I agree, Orac, these researchers have a sense of humor, as well as an intelligent study design. Next question ... are the muffins tasty? Can you add blueberries? Can you make them low-fat, low-cal? Will some pharma company snap this up and start passing out flaxseed muffins with their pens? Better than greasy chinese any day.
ReplyDeleteMy mom died of estrogen-dependent breast cancer. I'm a vegetarian woman who eats flaxseed as an egg substitute in baked goods. (1 tablespoon flaxseed "flour" to 3 tablespoons water, mixed till it "gells", per "egg" in the recipe).
ReplyDeleteI have to say I was unaware of its estrogen-suppressing properties. But then again I also eat plenty of tofu, which contains phytoestrogens....
Since I am not yet menopausal (I'm 38), is there likely to be any harm in increasing my flax intake to at least the levels tested in the study? I'm not likely to get pregnant.
Only a few days ago, the Danish Medicines Agency, which gives sales permissions for medicine, made a list of 155 health products that was sold as having medical effects, yet wasn't cathegorized as medicine. Many of them were said to be good against cancer.
ReplyDeleteSelling the products this way is a clear breach of Danish laws, and the sale of the products have now been made illegal (in some cases pending while a appeal of the decision is evaluated).
For a full list of the products, see here (sadly the product list is in Danish, so unless it's a brand name, it probably won't tell you much).
Really interesting study.
ReplyDeleteOf course, if this is proven to work in a larger study, it won't be "alternative" any more.
Nice entry! "Muffin intake" - hilarious :D
ReplyDelete-Ali
Not quite as funny as this:
ReplyDeletePubmed: Borden KL. 2000 http://tinyurl.com/b3ocz
"In all three cases, it appears that an intact zinc-binding site 1, but not site 2, is required for association. The sequence is referred to as a FRODO, named after the most famous RING bearer (Kentsis & Borden, 2000; Tolkein, 1954)…. Ultimately, a greater number of non-RING binding sequences will be necessary, as will further experimentation, to assess if FRODOs will be major players in RING interactions."
http://www.cafemadbard.com/tdb/plink/56/
This is NOT the first double blind, placebo-controlled muffin study. See this study published in Feb. 2004 http://www.ajcn.org/cgi/content/full/79/2/318
ReplyDelete"The women were randomly assigned to 3 treatment groups in which the daily diet was supplemented with either a placebo muffin (n = 15), a flaxseed muffin (n = 16), or a soy muffin (n = 15) for 16 wk. Fasting blood samples and 24-h urine samples were collected, and 3-d food records were recorded at baseline (week 0) and at the endpoint (week 16). The subjects were asked to record their muffin ingestion on daily diary cards and to return uneaten muffin portions. Compliance measured by this means was similar for all the treatment groups. The muffins were well tolerated, with compliance calculated to be 96%."
There seems to be a Canadian muffin connection. These researchers are sharing recipies:
"The placebo muffin was prepared with whole-wheat flour, instead of white flour, to raise the fiber content of the placebo muffin closer to that of the other muffins. Wheat fiber has been shown to have no significant effect on urinary estrogen metabolites (43). All muffins were formulated in an attempt to make them isocaloric and equivalent in macronutrients (fat, protein, and fiber). Hence, additional canola oil was added to the placebo (10 g) and soy muffins (4 g) but not to the flaxseed muffin. Muffins were also flavored with nutmeg, cinnamon, and vanilla extract to help maintain subject blindness. To maintain the double-blind status of the study, muffins were packaged in opaque wrappings with 7 muffins to a tray so that the different muffins could not be visually distinguished, and the muffins were labeled with a unique 4-digit number before delivery to the research assistant. For each subject visit, the research assistant received a list indicating which 4 trays of prewrapped muffins were to be dispensed to the subject for that 4-wk period."
Canada, world leader in Muffin Science.
Egads. It sounds like it might be a Phase I muffin study!
ReplyDeleteOdd how stud and study are nearly polar opposites. And how good science can be so clear and accessible. A negligible nit: if muffins were stored at 20 C (68 F), they wouldn't need defrosting.
ReplyDeleteBut Bob, flaxseed oil is not stable, so freezing preserves its value over the course of the study. Freshly ground flax and flax baked goods are lovely; the same muffin a week later is, well, not. And rancid muffin compliance would be much lower, no?
ReplyDelete